CHEMICAL SUBSTANCE DATASHEET
CHEMICAL SUBSTANCE IDENTIFICATION | |
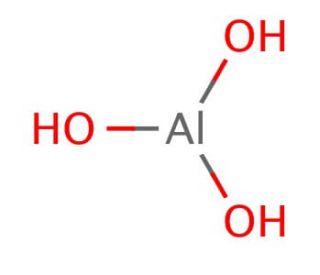
Chemical name | Aluminium Hydroxide [1] |
Synonyms | Alumina Hydrate, Aluminium hydrate, Hydrated alumina, etc [1] |
IUPAC name | aluminium(3+) trihydroxide [1] |
CAS No | 21645-51-2 |
REACH registration number | fully registered |
EC No | 244-492-7 |
Molecular formula | AlH3O3 |
Substance group/chemical family | mono-constituent substance |
Appearance Physical state Odour Form Colour | solid at 20°C and 1013 hPa [1] odourless powder white |
USES AND HANDLING ISSUES | |
Relevant identified uses | It is a compound with many biomedical applications: as a gastric antacid, an antiperspirant, in dentifrices, as an emulsifier, as an adjuvant in bacterins and vaccines, in water purification, etc. [2] Ingredient in household products [2] cables, textiles like carpets, epoxy casting resins, different applications in the chemical industry, SMC/ BMC as well as latex [3] |
Handling considerations | storage between +5°C and +30°C [4] |
PHYSICO-CHEMICAL PROPERTIES | |
Molecular weight | 78.004 g/mol [1, 2] |
Bulk density/Specific gravity | 2.42 g/cm³ at 20°C [1, 2] |
pH | 8 - 9 (100 g/l, H₂O, 20 °C) (slurry) [3] |
Particle size |
|
EC |
|
Melting point | 300 °C at 101 325 Pa [1, 2] |
Boiling point | 2 980 °C at 101 325 Pa [1] |
Flash point | not applicable |
Flammability | non flammable, based upon the use of this substance as a flame retardant in a number of industrial and consumer products and its chemical structure and known physical-chemical properties, the substance is known not to be flammable. [1] flame retardance (dehydration at 200°C) [3] |
Vapour density |
|
Vapour pressure | <0.1 hPa (20 °C) [4] |
Solubility in water | poorly soluble, with a water solubility of 0.00009 g/L at 20 °C. [1] |
Solubility in organic solvents | Not applicable because the substance is inorganic |
Solubility in inorganic solvents |
|
Hydrolysis |
|
Ionicity in water |
|
Surface tension |
|
Dispersion properties |
|
Explosiveness | it is not considered to be explosive [1] |
Other properties | it is not considered to have any oxidising properties [1] Hardness 3 (Mohs) [3] thermal expansion: 15*10-6/K (at T 20-300 °C) [3] |
Stability and reactivity | |
Chemical stability |
|
Reactivity hazards |
|
Corrosivity |
|
Polimerization |
|
Incompatibility with various substances |
|
Special remarks on reactivity |
|
Physical, chemical and biological coefficient | |
Koc |
|
Kow |
|
pKa |
|
log Kp |
|
Henry-constant |
|
ENVIRONMENTAL FATE AND BEHAVIOUR | |
Artificial pollution sources |
|
General terrestrial fate |
|
General aquatic fate |
|
General atmospheric fate |
|
General persistence and degradability |
|
Abiotic degradation and metabolites |
|
Biodegradation and metabolites | For an inorganic substance for which the chemical assessment is based on the elemental concentration, biotic degradation is an irrelevant process. [1] |
Bioconcentration | The available evidence shows the absence of aluminium biomagnification across trophic levels both in aquatic and terrestrial food chains. The existing information suggests not only that aluminium does not biomagnify, but rather that it tends to exhibit biodilution at higher trophic levels in the food chain. [1] |
Volatilization |
|
Photolysis |
|
Hydrolysis |
|
Soil adsorption and mobility |
|
ENVIRONMENTAL CONCENTRATIONS | |
Measured data |
|
ECOTOXICOLOGICAL INFORMATION | |
General adverse effects on ecosystem | |
Acute toxicity (LC50, EC50) | |
Aquatic systems |
|
Terrestrial systems |
|
Chronic toxicity (NOEC, LOEC) | |
Aquatic systems |
|
Terrestrial systems |
|
HUMAN HEALTH EFFECTS and PROTECTION | |
Routes of human exposures |
|
General effects |
|
Endocrine disruption |
|
Mutagenicity | No adverse effect observed [1] |
Carcinogenicity | Based on the weight of evidence approach for carcinogenicity no classification is required for aluminium hydroxide according to DSD (67/548/EEC) or CLP (1272/2008/EC) classification criteria. [1] |
Reprotoxicity | Based on the read-across from aluminium compounds for the toxicity to reproduction or development of aluminium hydroxide, no classification is required according to DSD (67/548/EEC) or CLP (1272/2008/EC) classification criteria. [1] |
Teratogenicity | No adverse effect observed [1] |
Skin, eye and respiratory irritations | Aluminium hydroxide is unlikely to lead to skin, eye or respiratory irritative effects. [1] The available human evidence is limited in amount but supports a low sensitisation potential for aluminium hydroxide. [1] |
Metabolism: absorption, distribution & excretion |
|
Exposure limits | TLV (inhalable fraction): 4 mg/m3; TLV (respirable fraction): 1.5 mg/m3; pregnancy risk group: D [2] |
Drinking water MAC |
|
Other information | There are no studies available on the repeated dose toxicity of aluminium hydroxide by the oral, inhalation and dermal route. [1] Overall, based on the read-across from aluminium compounds for the neurotoxicity of aluminium hydroxide, no classification is required according to DSD (67/548/EEC) or CLP (1272/2008/EC) classification criteria. [1] |
Animal toxicity data | |
Acute toxicity (LD50) | It is recommended that aluminium hydroxide is not to be classified for acute oral, dermal and inhalation toxicity. [1] Oral LD50 (rat) > 2000 mg/kg bw [1] Inhalation LC50 (rat) > 2.3 mg/L [1] |
Chronic toxicity (NOEL, LOEL) | |
ENVIRONMENTAL STANDARDS AND REGULATIONS | |
EINECS regulation | ̵ |
OSHA regulations etc. |
|
|
|
OTHER INFORMATION, SPECIAL REMARKS | |
Classification and proposed labelling with regard to toxicological data | No signal word [1] As a result of the hazard assessment and PBT/vPvB assessment it is found that substance aluminium hydroxide (CAS# 21645-51-2) does not meet the criteria for classification as hazardous (according to Directives 67/548/EEC and 1272/2008/EC) nor is it considered to be a PBT/vPvB. An exposure assessment and the subsequent step of risk characterisation is not required. [1] |
|
|
CREATED, LAST UPDATE | |
Created | 2019. 09. 04 |
Last update | 2019. 09. 11 |
REFERENCES | |
[1] ECHA, Aluminium hydroxide https://echa.europa.eu/hu/registration-dossier/-/registered-dossier/15529, Accessed 2019.09.04 [2] PUBCHEM, Aluminum Hydroxide (Compound), https://pubchem.ncbi.nlm.nih.gov/compound/Aluminum-hydroxide#section=Computed-Properties, Accessed 2019.09.05 [3] The mineral engineers, Aluminium hydroxide http://www.hpfminerals.kr/en/product-overview/aluminium-hydroxide, Accessed 2019.09.11 [4] Safety Data Sheet for Aluminium-hydroxide 101091http://www.merckmillipore.com/HU/hu/product/msds/MDA_CHEM-101091?Origin=PDP, Accessed 2019.09.11 | |