CHEMICAL SUBSTANCE DATASHEET
|
CHEMICAL SUBSTANCE IDENTIFICATION |
|
|
Chemical name |
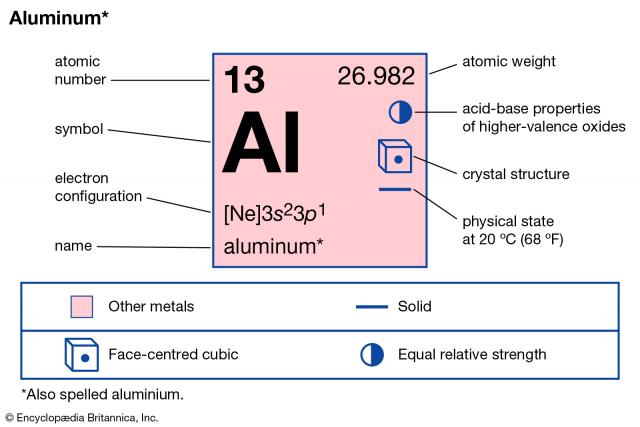
Aluminium [1] |
|
Synonyms |
Aluminum, Aluminum metal, etc [2] |
|
IUPAC name |
alumane [1], aluminium [2] |
|
CAS No |
7429-90-5 [1] |
|
REACH registration number |
fully registered [1] |
|
EC No |
231-072-3 [1] |
|
Molecular formula |
AlH3 [1], Al [2] |
|
Substance group/chemical family |
mono-constituent substance/element [1] |
|
Appearance Physical state Odour Form Colour |
solid @ 20°C and 1013 hPa odourless malleable, ductile metal; cubic crystal silver white [1] |
|
USES AND HANDLING ISSUES |
|
|
Relevant identified uses |
Aluminum is used in transportation (automobiles, airplanes, trucks, railcars, marine vessels, etc.), packaging (cans, foil, etc.), construction (windows, doors, siding, etc), consumer durables (appliances, cooking utensils, etc.), electrical transmission lines, machinery, and many other applications. Aluminum is often mixed with small amounts of other metals to form aluminum alloys, which are stronger and harder. Aluminum compounds have many different uses, for example, as alums in water-treatment and alumina in abrasives and furnace linings. They are also found in consumer products such as antacids, astringents, buffered aspirin food additives, and antiperspirants. Medicinally, aluminum and its salts are used in antacids, antidiarrheals, and protective dermatological pastes. It is also found in cosmetics and deodorants. |
|
Handling considerations |
Prevent formation of dust and wear appropriate personal protective equipment to minimise exposure when handling powders. |
|
PHYSICO-CHEMICAL PROPERTIES |
|
|
Molecular weight |
26.981538 g/mol [2] |
|
Bulk density/Specific gravity |
A weight-of-evidence approach was used to address this endpoint. The values obtained from 13 sources ranged from 2.5 - 2.9. [1] |
|
pH |
|
|
Particle size |
|
|
EC |
|
|
Melting point |
660 °C @ 101 325 Pa [1] A weight-of-evidence approach was used to address this endpoint. The values obtained from 16 sources ranged from 482 - 660 °C. [1] |
|
Boiling point |
2 450 °C at 101 325 Pa [1] A weight-of-evidence approach was used to address this endpoint. The values obtained from thirteen sources ranged from 2327 - 2767 C. [1] |
|
Flash point |
the study does not need to be conducted because the substance is inorganic [1] |
|
Flammability |
Fine powder or dust may ignite if exposed to a naked flame or other ignition source. [1,2] Highly flammable (67%), Not classified based on GHS criteria (33%) [3] Combustible Solid, finely divided dust is easily ignited [2] It doesn´t show a self-ignition behaviour. [1] |
|
Vapour density |
|
|
Vapour pressure |
13 hPa @ 974 °C [1] All available data sources agree that aluminium metal has a very low vapour pressure. [1] |
|
Solubility in water |
0-20 g/L @ 20 °C, pH 6.7 - 6.9 [1, 3] All available sources agree that aluminum metal is insoluble in water. [1] |
|
Solubility in organic solvents |
the study does not need to be conducted because the substance is inorganic [1] |
|
Solubility in inorganic solvents |
Soluble in dilute hydrochloric acid [2] |
|
Hydrolysis |
|
|
Ionicity in water |
|
|
Surface tension |
900 dynes/cm at 700°C (for molten aluminium) [1] |
|
Dispersion properties |
|
|
Explosiveness |
non explosive [1] |
|
Other properties |
non oxidising [1] Substance incapable of reacting exothermically with combustible materials. [1] |
|
Stability and reactivity |
|
|
Chemical stability |
Stable under normal temperatures. [1] Reacts with dilute HCl, H2SO4, KOH and NaOH with evolution of hydrogen. [2] |
|
Reactivity hazards |
Reacts with acids, alkalis with generation of flammable gas. [1] |
|
Corrosivity |
|
|
Polimerization |
|
|
Incompatibility with various substances |
|
|
Special remarks on reactivity |
|
|
Physical, chemical and biological coefficient |
|
|
Koc |
|
|
Log Kd |
3-5 [1] |
|
Kow |
the study does not need to be conducted because the substance is inorganic [1] |
|
pKa |
|
|
log Kp |
|
|
Henry-constant |
Not mandatory under REACH for aluminium. [1] |
|
ENVIRONMENTAL FATE AND BEHAVIOUR |
|
|
Artificial pollution sources |
It does not occur free in nature. [2] Aluminium is released to the environment both by natural processes and from anthropogenic sources. It is highly concentrated in soil-derived dusts from such activities as mining and agriculture, and in particulate matter from coal combustion. Aluminium silicates (clays), a major component of soils, contribute to the aluminium levels of dust. Mobilization of aluminium through human actions is mostly indirect and occurs as a result of emission of acidifying substances. In general, decreasing pH results in an increase in mobility and bioavailability for monomeric forms of aluminium. The most important raw material for the production of aluminium is bauxite, which contains up to 55% alumina (aluminium oxide). Direct anthropogenic releases of aluminium compounds are primarily to the atmosphere and are associated with industrial processes such as smelting. However, the use of aluminium and aluminium compounds in processing, packaging, storage of food products and as flocculants in the treatment of drinking-water may contribute to its presence in drinking-water and food stuffs. [4] |
|
General terrestrial fate |
Several factors influence aluminium mobility and subsequent transport within the environment. These include chemical speciation, hydrological flow paths, soil-water interactions, and the composition of the underlying geologicalmaterials. The solubility of aluminium in equilibrium with solid phase Al(OH)3 is highly dependent on pH and on complexing agents such as fluoride, silicate, phosphate and organic matter. The chemistry of inorganic aluminium in acid soil and stream water can be considered in terms of mineral solubility, ion exchange and water mixing processes.Upon acidification of soils, aluminium can be released into solution for transport to streams. Mobilization of aluminium by acid precipitation results in more aluminium being available for plant uptake. [4] |
|
General aquatic fate |
Several factors influence aluminium mobility and subsequent transport within the environment. These include chemical speciation, hydrological flow paths, soil-water interactions, and the composition of the underlying geologicalmaterials. The solubility of aluminium in equilibrium with solid phase Al(OH)3 is highly dependent on pH and on complexing agents such as fluoride, silicate, phosphate and organic matter. The chemistry of inorganic aluminium in acid soil and stream water can be considered in terms of mineral solubility, ion exchange and water mixing processes.Upon acidification of soils, aluminium can be released into solution for transport to streams. Mobilization of aluminium by acid precipitation results in more aluminium being available for plant uptake. [4] |
|
General atmospheric fate |
|
|
General persistence and degradability |
|
|
Abiotic degradation and metabolites |
In general, (abiotic) degradation is an irrelevant process for inorganic substances that are assessed on an elemental basis. [1] |
|
Biodegradation and metabolites |
According to the guidance given in Annex VII of REACH legislation and Chapter R.7B (Endpoint Specific Guidance) of the ECHA REACH Guidance Document, (2008), the requirements for “Ready biodegradability” can be waived if the substance is inorganic. [1] |
|
Bioconcentration |
The available evidence shows the absence of aluminium biomagnification across trophic levels both in aquatic and terrestrial food chains. The existing information suggests not only that aluminium does not biomagnify, but rather that it tends to exhibit biodilution at higher trophic levels in the food chain. [1] |
|
Volatilization |
|
|
Photolysis |
Not mandatory under REACH for this substance. [1] |
|
Hydrolysis |
Aluminium can participate in hydrolysis reactions, thereby forming a number of monomeric and polymeric Al-hydroxides and this process is highly dependent on pH. Under REACH (ECHA 2008, Chapter R.7B – Endpoint Specific Guidance), the term ‘Hydrolysis’ refers to the “Decomposition or degradation of a chemical by reaction with water”, and this is a function of pH (i. e., abiotic degradation). Characterization of aluminium in environmental media is typically based on total aluminium concentrations inclusive of all specific chemical forms or species. Since hydrolysis changes the chemical form but does not decompose aluminium and since characterization of total aluminium considers all chemical forms, the concept of degradation of aluminium by hydrolysis is not relevant in the consideration of its environmental fate. [1] |
|
Soil adsorption and mobility |
The potential for adsorption is low at different pH-levels. Approximately 8% of the total aluminium was bound to particles which was further reduced to < 1% at pH-values > 7 (at soft and hard water). Log Kd-values of 3 - 5 were derived. The potential of aluminium for adsorption to sediment and soil particles is mainly driven by its speciation and the concentration of dissolved organic carbon (DOC). Aluminium speciation is very complex and changes significantly with the pH.In the absence of organic matter, Al3+is the predominant aluminium species at low pH (less than 5.5). As pH increases above 5.5, aluminium-hydroxide complexes formed by hydrolysis become increasingly important and dominate aqueous aluminium speciation. The presence of a moderate amount of organic matter in soft water results in organically complexed aluminium being the dominant aluminium form when the pH is between 4 and 7. Above pH 7, anionic aluminium hydroxide predominates. The DOC and the pH are also affecting the adsorption and desorption of aluminium to organic particles. For evaluation of adsorption at different pH-levels a chemical simulation was performed. [1] |
|
ENVIRONMENTAL CONCENTRATIONS |
|
|
Measured data |
In urban areas aluminium levels in street dust range from 3.7 to 11.6 µg/kg. Airborne aluminium levels vary from 0.5 ng/m3 over Antarctica to more than 1000 ng/m3 in industrialized areas. Dissolved aluminium concentrations for water in the circumneutral pH range are usually quite low, ranging from 1.0 to 50 µg/litre. This rises to 500-1000 µg/litre in more acidic water. At the extreme acidity of water affected by acid mine drainage, dissolved aluminium concentrations of up to 90 mg/litre have been measured. [4] |
|
ECOTOXICOLOGICAL INFORMATION |
|
|
General adverse effects on ecosystem |
|
|
Acute toxicity (LC50, EC50) |
|
|
Aquatic systems |
There is little or no evidence that aluminium metal, metal powders or metal oxides have ever resulted in aquatic toxicity effects.[1] Al Chloride (soluble salt) was not classified by the C&L Committee in 1999 and therefore less soluble forms of Al would also logically not classify. |
|
Terrestrial systems |
Aluminium, aluminium powders and aluminium oxide are non hazardous (not classified for the environment). Aluminum (Al) is the most commonly occurring metallic element, comprising eight percent of the earth's crust and is therefore found in great abundance in both the terrestrial and sediment environments. Concentrations of 3-8% (30,000-80,000 ppm) are not uncommon. The relative contributions of anthropogenic aluminium to the existing natural pools of aluminium in soils and sediments is very small, and therefore, not relevant either in terms of added amounts or in terms of toxicity. Based on these exposure considerations, additional sediment and/or soil testing is not warranted. [1] |
|
Chronic toxicity (NOEC, LOEC) |
|
|
Aquatic systems |
There is little or no evidence that aluminium metal, metal powders or metal oxides have ever resulted in aquatic toxicity effects.[1] Al Chloride (soluble salt) was not classified by the C&L Committee in 1999 and therefore less soluble forms of Al would also logically not classify. |
|
Terrestrial systems |
Aluminium, aluminium powders and aluminium oxide are non hazardous (not classified for the environment). Aluminum (Al) is the most commonly occurring metallic element, comprising eight percent of the earth's crust and is therefore found in great abundance in both the terrestrial and sediment environments. Concentrations of 3-8% (30,000-80,000 ppm) are not uncommon. The relative contributions of anthropogenic aluminium to the existing natural pools of aluminium in soils and sediments is very small, and therefore, not relevant either in terms of added amounts or in terms of toxicity. Based on these exposure considerations, additional sediment and/or soil testing is not warranted. [1] |
|
HUMAN HEALTH EFFECTS and PROTECTION |
|
|
Routes of human exposures |
inhalation, dermal, oral, eye exposure |
|
General effects |
irritation eyes, skin, respiratory system [2] |
|
Endocrine disruption |
|
|
Mutagenicity |
The read-across from aluminium compounds within a weight of evidence approach does not support a systemic mutagenic hazard for aluminium or aluminium metal. [1] Based on the read-across from aluminium compounds within a weight of evidence approach (GLP-guideline studies) for genetic toxicity, no classification is required according to DSD (67/548/EEC) or CLP (1272/2008/EC) classification criteria. [1] |
|
Carcinogenicity |
Based on the weight of evidence approach for carcinogenicity no classification is required for aluminium metal according to DSD (67/548/EEC) or CLP (1272/2008/EC) classification criteria.[1] The carcinogenic risk from aluminium and its compounds has not been evaluated by IARC. However, IARC has deemed that that there is sufficient evidence to show that certain exposures occurring during the production of aluminium cause cancer in humans; therefore “aluminium production” has been classified as carcinogenic to humans (Group I) by IARC. [5] |
|
Reprotoxicity |
There is no information available on the toxicity to reproduction or development of metal. [1] In terms of hazard assessment of toxic effects, available data on the toxicity to reproduction/development of other aluminium compounds was taken into account by read-across following a structural analogue approach, since the pathways leading to toxic outcomes are likely to be dominated by the chemistry and biochemistry of the aluminium ion (Al3+) [1]. Based on the read-across from aluminium compounds for toxicity to reproduction or development, no classification is required according to DSD (67/548/EEC) or CLP (1272/2008/EC) classification criteria. [1] |
|
Teratogenicity |
Based on the read-across from aluminium compounds for toxicity to reproduction or development, no classification is required according to DSD (67/548/EEC) or CLP (1272/2008/EC) classification criteria. [1] |
|
Skin, eye and respiratory irritations |
Available data are adequate to conclude that there is no need to recommend Classification and Labelling (2008) requirements for skin irritation from acute exposures to aluminium metal, aluminium oxide and aluminium hydroxide dust and powder. [1] For aluminium metal dust or powder that undergoes immediate oxidation to form the oxide on contact with air, the data are adequate to conclude that there is not need to recommend Classification and Labelling (2008) requirements for eye irritation from acute exposures. [1] The weight of evidence from human and animal studies does not support a substance-specific acute, respiratory irritative hazard for aluminium dust or powder. Overall, according to DSD (67/548/EEC) or CLP (1272/2008/EC) classification criteria for irritation/corrosion, no classification is required. [1] The current weight of evidence supports a low skin and respiratory sensitizing potential for the poorly soluble substance aluminium metal. Overall, according toDSD (67/548/EEC) or CLP (1272/2008/EC) classification criteria for sensitisation, no classification is required. [1] |
|
Metabolism: absorption, distribution & excretion |
Aluminum and its compounds appear to be poorly absorbed in humans. The mechanism of gastrointestinal absorption of aluminum has not yet been fully elucidated. The highest levels of aluminum may be found in the lungs, where it may be present as inhaled insoluble particles. The urine is the most important route of aluminum excretion. [2] |
|
Exposure limits |
Workers (inhalation, systemic effects, long term exposure) DNEL (Derived No Effect Level): 3.72 mg/m³ [1,3] Workers (inhalation, local effects, long term exposure) DNEL (Derived No Effect Level): 3.72 mg/m³ [1,3]
|
|
Drinking water MAC |
EPA 50-200 ug/L (Federal drnking water guidelines) |
|
Other information |
Overall, there is no consistent evidence for neurotoxicity of Al-compounds after repeated exposure. In particular the substances of low bioavailability, such as Al-metal, Al2O3 and Al(OH)3 are unlikely to cause neurotoxic effects because of their limited bioavailability. No classification is required according to DSD (67/548/EEC) or CLP (1272/2008/EC) classification criteria. Overall, there is no consistent evidence for neurotoxicity of Al-compounds after repeated exposure. [1] |
|
Animal toxicity data |
|
|
Acute toxicity (LD50) |
According to DSD (67/548/EEC) or CLP (1272/2008/EC) classification criteria for acute toxicity, no classification is warranted. [1] Aluminium metal (dust/powder) is not to be classified for acute oral, inhalation and dermal toxicity. [1] Oral LD50 (rat) > 2000 mg/kg bw [1] Oral LD50(rat): 15 900 mg/kg bw [3] Inhalation LC50 (rat) (4 h) > 888 mg/m³ [1,3] |
|
Chronic toxicity (NOEL, LOEL) |
According to DSD (67/548/EEC) or CLP (1272/2008/EC) classification criteria for repeated dose toxicity, no classification is required. [1] There are no studies available on the repeated dose toxicity of aluminium metal by the oral, inhalation and dermal route. In terms of hazard assessment of toxic effects, available data on the repeated dose toxicity of other aluminium compounds was taken into account by read-across following a structural analogue approach, since the pathways leading to toxic outcomes are likely to be dominated by the chemistry and biochemistry of the aluminium ion (Al3+) [1] Based on read-across Oral: NOAEL (chronic, rat) 30 mg Al/kg bw/day as aluminium citrate [1] Inhalation: LOAEC (subchronic, rat) 50 mg Al/m³ as aluminium powder [1,3]
Repeated dose toxicity, oral NOAEL (rat): 200 - 3 225 mg/kg bw/day [3] Repeated dose toxicity, oral NOAEL (rat): 141 - 302 mg/kg diet [3] Repeated dose toxicity, oral NOAEL (dog): 1 034 - 1 087 mg/kg bw/day [3] Repeated dose toxicity, oral NOAEL (dog): 90 mg/kg diet [3] |
|
ENVIRONMENTAL STANDARDS AND REGULATIONS |
|
|
REACH/CLP |
Danger! According to the classification provided by companies to ECHA in REACH registrations this substance is a flammable solid and in contact with water releases flammable gases. Additionally, the classification provided by companies to ECHA in CLP notifications identifies that this substance catches fire spontaneously if exposed to air. This substance is covered by several Harmonised Classifications and Labelling's (CLH) entries approved by the European Union. Differentiating between the different CLH's entries requires manual verification. [3]
According to REACH registrations: [3] H261: In contact with water releases flammable gas H228: Flammable solid not classified According to some CLP notifications: [3] H261: In contact with water releases flammable gas H228: Flammable solid H250: Catches fire spontaneously if exposed to air not classified |
|
EINECS regulation |
̵listed |
|
NIOSH regulations etc. |
Recommended Exposure Limit: 10-Hr Time-Weighted Avg: 10 mg/cu m (total). [2] Recommended Exposure Limit: 10 Hr Time-Weighted Avg: 5 mg/cu m (resp). [2] Recommended Exposure Limit: 10 Hr Time-Weighted Avg: 2 mg/cu m. /Aluminum (soluble salts and alkyls, as Al) [2] Recommended Exposure Limit: 10 Hr Time-Weighted Avg: 5 mg/cu m. /Aluminum (pyro powders and welding fumes, as Al [2] |
|
|
|
|
OTHER INFORMATION, SPECIAL REMARKS |
|
|
Classification and proposed labelling with regard to toxicological data |
|
|
|
|
|
CREATED, LAST UPDATE |
|
|
Created |
2020.06.06 |
|
Last update |
2020.06.18 |
|
REFERENCES |
|
|
[1] ECHA, Aluminium https://echa.europa.eu/hu/registration-dossier/-/registered-dossier/15572, Accessed 2020.06.08 [2] PubChem, Aluminium https://pubchem.ncbi.nlm.nih.gov/compound/Aluminum, Accessed 2020.05.30 [3] ECHA, Aluminium, Brief profile https://echa.europa.eu/hu/brief-profile/-/briefprofile/100.028.248, Accessed 2020.06.08 [4] World Health Organization, Geneva (1997) Environmental health criteria ; 194, Aluminium, ISBN 92 4 157194 2, ISSN 0250-863X Avalable from: http://www.inchem.org/documents/ehc/ehc/ehc194.htm#SectionNumber:1.1, Accessed: 2020.06.18 [5] IARC (International Agency for Research on Cancer) (1987) Overall Evaluation of Carcinogenicity: An Updating of IARC Monographs (Volumes 1 to 42). Lyon, France: World Health Organization, International Agency for Research on Cancer. |
|