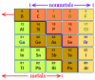
“Metalloids, are chemical elements with properties intermediate between those of typical metals and nonmetals. Usually considered under this classification are the chemical elements boron, silicon, germanium, arsenic, antimony, and tellurium. The rare elements polonium and astatine are also sometimes included. Most of these elements are important industrial materials, being used to make transistors and other semiconductor devices, ceramics, solar batteries, and certain polymers. Metalloids are usually brittle, somewhat shiny solids that behave as electrical insulators at room temperature but become comparable to metals as electrical conductors when heated or when small quantities of certain elements are introduced into the lattices of their crystalline structures. Metalloids have electronic structures intermediate between the nearly empty outer electron shells of the typical metals and the nearly filled electron shells of the nonmetals. Thus, they have enough empty electron orbitals (pathways within the shells) into which electrons can be moved to conduct electric current. Their chemical properties are intermediate between the behaviour of electropositive and electronegative atoms” (Britannica, 2018).
In our database the following elements are classified as metalloids: boron (B), silicon (Si), germanium (Ge)*, arsenic (As), antimony (Sb)*, tellurium (Te), polonium (Po) and astatine (At).
* on the 2017 CRMs (27) list
References:
Britannica, Available from: https://www.britannica.com/science/metalloid, Accessed: 21.06.2018
CRMs (27) (2017) COMMUNICATION FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS on the 2017 list of Critical Raw Materials for the EU
Photo: https://www.chemicool.com/definition/metalloid.html