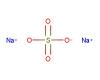
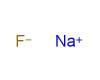
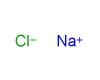
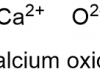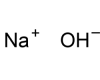Alkali metals belong to the elements in group one of the periodic table (with the exception of hydrogen). This group includes the following elements: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). These elements form alkaline solutions when they react with water and they have just one valence electron, which means that they form only weak metallic bonds. As a result, they are relatively soft and have low melting points. Because the single valence electron is easily lost, these metals are highly reactive. They react vigorously with both air and water. Alkali metals also readily combine with the elements of group seventeen of the periodic table (chlorine, fluorine, bromine etc.) to form stable ionic compounds like sodium chloride.
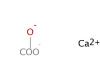
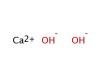
The alkaline earth metals belong to group two of the periodic table which comprises the elements beryllium (Be)*, magnesium (Mg)*, calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements in this group are all shiny and silvery-white in appearance. All of the elements of group two have two electrons in their outer shell. Metallic bonds in the alkaline earth metals are thus stronger than for the alkali metals, resulting in higher melting points, but they are still quite reactive because the two outer electrons are easily lost. As a result, they are not found in nature in their elemental state. All but one of the alkaline earth metals react with the halogens (chlorine, fluorine etc.) to form ionic compounds (beryllium chloride is the exception, because the bonding is covalent). All of the alkaline earth metals except beryllium and magnesium also react with water to produce hydrogen gas and their respective hydroxides (magnesium will react with steam, however). Essentially, the heavier the alkaline earth metal, the more vigorously it will react with water. All of the alkaline earth metals, except magnesium and strontium, have at least one naturally occurring radioisotope: beryllium-7, beryllium-10, and calcium-41 are trace radioisotopes. Calcium-48 and barium-130 have very long half-lives and thus occur naturally. All isotopes of radium are radioactive.
*on the 2017 CRMs (27) list
References:
Wells, Christopher J. (2017) TechnologyUKnet, Alkali and Alkaline Earth Metals, Available from: https://www.technologyuk.net/physics/matter/alkali-and-alkali-earth-metals.shtml, Accessed: 20th of June, 2018
Science of Everyday Things, Alkaline-earth metals, Available from: https://www.encyclopedia.com/science-and-technology/chemistry/compounds-and-elements/alkaline-earth-metals, Accessed: 20th of June 2018
CRMs (27) (2017) COMMUNICATION FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS on the 2017 list of Critical Raw Materials for the EU