CHEMICAL SUBSTANCE DATASHEET
|
CHEMICAL SUBSTANCE IDENTIFICATION |
|
|
Chemical name |
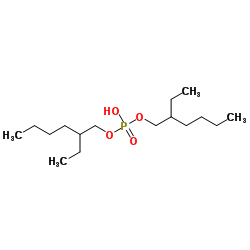
Di-(2-ethylhexyl)phosphoric acid (DEHPA or HDEHP) [2] |
|
Synonyms |
|
|
IUPAC name |
bis[(2-ethylhexyl)oxy]phosphinic acid [1] |
|
CAS No |
298-07-7 |
|
REACH registration number |
|
|
EC No |
206-056-4 |
|
Molecular formula |
C16H35O4P |
|
Substance group/chemical family |
Organic, Mono constituent substance |
|
Appearance Physical state Odour Form Colour |
Liquid (100%) at 20°C and 1013 hPa [1]
Odourless Viscous (100%) [1] |
|
USES AND HANDLING ISSUES |
|
|
Relevant identified uses |
extractant in manufacture of basic metals, including alloys, production of PUR foam, polymer preparations and compounds |
|
Handling considerations |
Precautions for safe handling: |
|
PHYSICO-CHEMICAL PROPERTIES |
|
|
Molecular weight |
322.42 g/mol [2] |
|
Bulk density/Specific gravity |
0.976 g/cm3 @ 20 °C [1] |
|
pH |
Strongly acidic [2] |
|
Particle size |
|
|
EC |
|
|
Melting point |
-50 °C at 101 325 Pa [1] |
|
Boiling point |
240 °C at 101 325 Pa [1] |
|
Flash point |
181 °C @ 101.3 kPa [1] |
|
Flammability |
Non flammable (100%) [1] |
|
Vapour density |
|
|
Vapour pressure |
0 Pa @ 25 °C [1] |
|
Solubility in water |
182 mg/L @ 20 °C [1] |
|
Solubility in organic solvents |
Soluble in benzene, hexane, and 4-methyl-2-pentanone [2] |
|
Solubility in inorganic solvents |
|
|
Hydrolysis |
|
|
Ionicity in water |
|
|
Surface tension |
52.24 mN/m @ 164 mg/L and 20 °C [1] |
|
Dispersion properties |
|
|
Explosiveness |
Non-explosive (100%) [1] |
|
Other properties |
Self-ingnition (Autoflammability) 255 °C @ 101.1 kPa [1] Non- oxidising (100%) [1] Dynamic viscosity at 20 °C: 40.99 mPa.s [1] |
|
Stability and reactivity |
|
|
Chemical stability |
The product is stable. [1] |
|
Reactivity hazards |
No specific test data related to reactivity available for this product or its ingredients. [1] |
|
Corrosivity |
Mildly corrosive to most metals [2] |
|
Polimerization |
|
|
Incompatibility with various substances |
No specific data. |
|
Special remarks on reactivity |
Avoid hydrogen formation: Keep away from alkaline solutions and non-noble metals (e.g. iron, zinc, aluminum) [1] |
|
Physical, chemical and biological coefficient |
|
|
Koc |
251 @ 20 °C [1] |
|
Kow |
2.88 @ 25 °C (log Kow) [1] |
|
pKa |
1.47 @ 25 °C [1] |
|
log Kp |
|
|
Henry-constant |
0.004 Pa m³/mol @ 25 °C [1] |
|
ENVIRONMENTAL FATE AND BEHAVIOUR |
|
|
Artificial pollution sources |
Bis(2-ethylhexyl) phosphate's production and use as a metal extraction agent, a textile lubricant and antistatic agent, an extreme pressure additive, an intermediate for wetting agents and detergent, and a feedstock for chemical synthesis may result in its release to the environment through various waste streams. [2] |
|
General terrestrial fate |
If released to soil, bis(2-ethylhexyl) phosphate is expected to have no mobility based upon an estimated Koc of 1.7X10+4. Volatilization from moist soil surfaces is not expected to be an important fate process based upon an estimated Henry's Law constant of 4.1X10-8 atm-cu m/mole. The pKa of bis(2-ethylhexyl) phosphate has been estimated as 1.47, indicating that this compound will primarily exist in anion form in the environment and anions generally do not adsorb more strongly to organic carbon than their neutral counterparts. The sorption of organophosphorus compounds in soil depends on both organic matter and clay content of soil and the sorption increases as the pH of soil decreases. In a study on the sorption of bis(2-ethylhexyl) phosphate on kaolinite and amectite, the acidic phosphoric group reacted rapidly and almost irreversibly with the surface cations of the clay mineral structure. Bis(2-ethylhexyl) phosphate is not expected to volatilize from dry soil surfaces based upon its vapor pressure. [2] |
|
General aquatic fate |
If released into water, bis(2-ethylhexyl) phosphate is expected to adsorb to suspended solids and sediment based upon the estimated Koc. Bis(2-ethylhexyl) phosphate, present at 100 mg/L, reached 0-17% of its theoretical BOD in 4 weeks using an activated sludge inoculum at 30 mg/L and the Japanese MITI test and therefore this compound is not expected to biodegrade rapidly in the environment. Volatilization from water surfaces is not expected to be an important fate process based upon this compound's estimated Henry's Law constant. Measured BCF values of 1-2.4 and 2.7-6.0 suggest bioconcentration in aquatic organisms is low. Hydrolysis is expected to be an important environmental fate process since this compound contains functional groups (esters) that hydrolyze under environmental conditions. The hydrolysis half-life of the analog dimethyl phosphoric acid in neutral solution at 100 °C was found to be 2.4 days. Increasing the carbon chain substituent on phosphoric acid (as in the case of bis(2-ethylhexyl) phosphate) may not increase the rate of hydrolysis. [2] |
|
General atmospheric fate |
If released to air, an estimated vapor pressure of 4.7X10-8 mm Hg at 25 °C indicates bis(2-ethylhexyl) phosphate will exist solely in the particulate phase in the atmosphere. Particulate-phase bis(2-ethylhexyl) phosphate will be removed from the atmosphere by wet or dry deposition. Bis(2-ethylhexyl) phosphate does not contain chromophores that absorb at wavelengths >290 nm and therefore is not expected to be susceptible to direct photolysis by sunlight. [2] |
|
General persistence and degradability |
|
|
Abiotic degradation and metabolites |
|
|
Biodegradation and metabolites |
Readily biodegradable (100%) [1] To test for its biodegradability potential, bis(2-ethylhexyl) hydrogen phosphate was incubated for 28 days in continuously stirred 250 ml closed flask in the dark with an inoculum originally collected from a local, predominantly municipal wastewater treatment plant. The applied method was the OECD Guideline 301 F. In this assay, biodegradation was estimated by biological oxygen demand (BOD) over time. BOD was measured daily by a manometer. The incubation temperature was 20 +/-1°C. The concentration of innoculum was 30 mg /L and the one of the substance was 100 mg/L. Degradation was calculated by substracting the amount BOD in the negative (innoculum only) control from that in the substance or positive control at any given time point and divided by the chemical oxygen demand (COD) or thereotical oxygen demand (ThOD). The 28-day degradation was 75% forbis(2-ethylhexyl) hydrogen phosphate and 82% for the positive control (with reference substance). A second test on ready biodegradability was conducted according to the national Japanese standard method comparable to the OECD Guideline 301 C (Ready Biodegradability: Modified MITI Test (I)). After 14 days, the substance showed 0-17% degradation realting to BOD, and 0% degradation relating to the test substance concentration. As recommended in Guidance R.7b (ECHA, 2012), in cases of conflicting test results, differences in stringency and the origin of the inoculum in order to check whether or not differences in the adaptation of the inoculum may be the reason are considered. The reported data are carefully checked and no differences in test design were found in both tests. The positive result in the well documented study for ready biodegradability is considered as indicative of rapid and ultimate degradation and the positive test result supersede the negative test result.[3] |
|
Bioconcentration |
BCF aquatic/sediment: 6 (dimensionless) [1] Therefore, bioconcentration in aquatic organisms is low. [2] |
|
Volatilization |
Volatilization from water surfaces is not expected to be an important fate process based upon this compound's estimated Henry's Law constant. Bis(2-ethylhexyl) phosphate is not expected to volatilize from dry soil surfaces based upon its vapor pressure. [2] |
|
Photolysis |
Bis(2-ethylhexyl) phosphate does not contain chromophores that absorb at wavelengths >290 nm and therefore is not expected to be susceptible to direct photolysis by sunlight. [2] |
|
Hydrolysis |
Hydrolysis is expected to be an important environmental fate process since this compound contains functional groups (esters) that hydrolyze under environmental conditions. The hydrolysis half-life of the analog dimethyl phosphoric acid in neutral solution at 100 °C was found to be 2.4 days. Increasing the carbon chain substituent on phosphoric acid (as in the case of bis(2-ethylhexyl) phosphate) may not increase the rate of hydrolysis. [2] |
|
Soil adsorption and mobility |
It is expected to have no mobility based upon an estimated Koc of 1.7X10+4 . This compound will primarily exist in anion form in the environment and anions generally do not adsorb more strongly to organic carbon than their neutral counterparts. The sorption of organophosphorus compounds in soil depends on both organic matter and clay content of soil and the sorption increases as the pH of soil decreases. [2] |
|
ENVIRONMENTAL CONCENTRATIONS |
|
|
Measured data |
|
|
ECOTOXICOLOGICAL INFORMATION |
|
|
General adverse effects on ecosystem |
|
|
Acute toxicity (LC50, EC50) |
|
|
Aquatic systems |
LC50: 30 mg/L (Freshwater fish) (4 days) [1] LC50: >56 mg/L (Danio rerio) (96 h) LC50: 34 mg/L (Oncorhynchus mykiss) (48 h) [2] LC50: 30 mg/L (Oncorhynchus mykiss) (96 h) [2] EC50 / LC50: 60.7 mg/L (freshwater invertebrates) (48 h) [1] LC50: 46.8 mg/L (Daphnia magna) (72 h) [2] LC50: >42 mg/L (Daphnia magna) (24 h) [2] LC50: >42 mg/L (Daphnia magna) (48 h) [2] LC50: 27.2 mg/L (Daphnia magna) (96 h) [2] EC50: 100 mg/L (freshwater algae) (72 h) [1] EC50: 890 mg/L (microorganisms) (3h) [1] |
|
Terrestrial systems |
|
|
Chronic toxicity (NOEC, LOEC) |
|
|
Aquatic systems |
EC10 / LC10 or NOEC: 20.6 mg/L(freshwater fish) (48 days) [1] EC10 or NOEC: 50 mg/L (freshwater algae) (72 h) [1] EC10 or NOEC: 196 mg/L (microorganisms) (3h) [1] |
|
Terrestrial systems |
|
|
HUMAN HEALTH EFFECTS and PROTECTION |
|
|
Routes of human exposures |
inhalation, dermal, oral, eye exposure |
|
General effects |
|
|
Endocrine disruption |
|
|
Mutagenicity |
|
|
Carcinogenicity |
|
|
Reprotoxicity |
No adverse effect observed NOAEL 150 mg/kg bw/day (subacute, rat) (effect on fertility) (oral route) [1] No adverse effect observed NOAEL 1 000 mg/kg bw/day (subacute, rat) (Effect on developmental toxicity) (oral route) [1] |
|
Teratogenicity |
|
|
Skin, eye and respiratory irritations |
Adverse effect observed (corrosive to skin, irritative to eyes) [1] |
|
Metabolism: absorption, distribution & excretion |
Low bioaccumulation potential [1] |
|
Exposure limits |
Inhalation: Long term: DNEL: 3.52 mg/m³ (workers, systemic effects) Inhalation: Long term: (DNEL) 870 µg/m³ (general population, systemic effects) Inhalation: Short term: DNEL: 3.52 mg/m³ (workers, systemic effects) Inhalation: Short term: DNEL: 870 µg/m³ (general population, systemic effects) Inhalation: Long term: DNEL: 1 mg/m³ (workers, local effects) Inhalation: Acute/short term: DNEL: 1 mg/m³ (workers, local effects) Dermal: Long term: 500 µg/kg bw/day (workers, systemic effects) Dermal: Long term: 250 µg/kg bw/day (general population, systemic effects) Dermal: Acute/short term: 500 µg/kg bw/day (workers, systemic effects) Dermal: Acute/short term: 250 µg/kg bw/day (general population, systemic effects) Oral: Long term: 250 µg/kg bw/day (general population, systemic effects) Oral: Acute/short term: 250 µg/kg bw/day (general population, systemic effects) Eye: Medium hazard (no threshold derived) [1] |
|
Drinking water MAC |
|
|
Other information |
|
|
Animal toxicity data |
|
|
Acute toxicity (LD50) |
LD50: 1 400 mg/kg bw (rat, oral) [1] LD50: 2 000 mg/kg bw (rabbit, dermal) [1] |
|
Chronic toxicity (NOEL, LOEL) |
NOAEL: 150 mg/kg bw/day (subacute, rat) (Oral route – repeated dose toxicity) [1] |
|
ENVIRONMENTAL STANDARDS AND REGULATIONS |
|
| REACH/CLP |
Danger! According to the classification provided by companies to ECHA in REACH registrations this substance causes severe skin burns and eye damage, is harmful if swallowed and causes serious eye damage. According to REACH registrations H318: Causes serious eye damage. H302: Harmful if swallowed. H314: Causes severe skin burns and eye damage Additionally, the classification provided by companies to ECHA in CLP notifications identifies that this substance is harmful in contact with skin and causes skin irritation. According to CLP notifications H318: Causes serious eye damage. H302: Harmful if swallowed. H3012: Harmful in contact with skin. H314: Causes severe skin burns and eye damage. H315: Causes skin irritation. H412: Harmful to aquatic life with long-lasting effects. |
|
EINECS regulation |
Listed on EINECS (European INventory of Existing Commercial chemical Substances) List |
|
OSHA regulations etc. |
|
|
OTHER INFORMATION, SPECIAL REMARKS |
|
|
Classification and proposed labelling with regard to toxicological data |
|
|
|
|
|
CREATED, LAST UPDATE |
|
|
Created |
2019. 11. 25 |
|
Last update |
2019. 11. 26 |
|
REFERENCES |
|
|
[1] ECHA, Bis(2-ethylhexyl) hydrogen phosphate, https://echa.europa.eu/hu/brief-profile/-/briefprofile/100.005.507 Accessed 2019.11.25 [2] PUBChem https://pubchem.ncbi.nlm.nih.gov/compound/Bis_2-ethylhexyl_-hydrogen-phosphate Accessed 2019.11.26 [3] ECHA, Bis(2-ethylhexyl) hydrogen phosphate, https://echa.europa.eu/hu/registration-dossier/-/registered-dossier/5599/5/3/2 Accessed 2020.05.10 |
|