CHEMICAL SUBSTANCE DATASHEET
|
CHEMICAL SUBSTANCE IDENTIFICATION |
|
|
Chemical name |
Ammonia, anhydrous |
|
Synonyms |
Ammonia anhydrous, nitrogen trihydride [1] |
|
IUPAC name |
ammonia [1] |
|
CAS No |
7664-41-7 |
|
REACH registration number |
|
|
EC No |
231-635-3 |
|
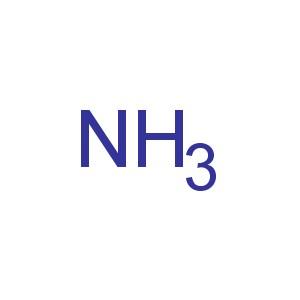
Molecular formula |
NH3 [1] |
|
Substance group/chemical family |
Mono constituent substance/ Inorganic [1] |
|
Appearance Physical state Odour Form Colour |
Gaseous (100%) at 20°C and 1013 hPa [1] or or compressed liquid (compressed under its own pressure) [2]
strong, pungent, suffocating odour [2]
clear colourless gas or liquid [2] |
|
USES AND HANDLING ISSUES |
|
|
Relevant identified uses |
This substance is used in the following products: adhesives and sealants, washing & cleaning products, coating products, cosmetics and personal care products, textile treatment products and dyes, pH regulators and water treatment products, non-metal-surface treatment products, water treatment chemicals, paper chemicals and dyes, metal surface treatment products, heat transfer fluids, pharmaceuticals, extraction agents and photo-chemicals. This substance has an industrial use resulting in manufacture of another substance (use of intermediates). This substance is used in the following areas: municipal supply (e.g. electricity, steam, gas, water) and sewage treatment. This substance is used for the manufacture of: chemicals, textile, leather or fur, pulp, paper and paper products, plastic products and fabricated metal products. This substance is used in the following activities or processes at workplace: transfer of chemicals, closed, continuous processes with occasional controlled exposure, transfer of substance into small containers, closed batch processing in synthesis or formulation, closed processes with no likelihood of exposure, batch processing in synthesis or formulation with opportunity for exposure, hand mixing with intimate contact only with personal protective equipment available and industrial spraying. [1] |
|
Handling considerations |
Prevention statementsWhen handling this substance: do not breathe the dust, fume, gas, mist, vapours or spray; keep away from heat, sparks, open flames and/or hot surfaces – No smoking; avoid release to the environment; wear protective gloves and/or clothing, and eye and/or face protection as specified by manufacturer/supplier. Response statementsIn case of incident: In case of leaking gas fire do not extinguish unless leak can be stopped safely. If on skin (or hair): take off immediately all contaminated clothing. Rinse skin with water or shower. If inhaled: remove victim to fresh air and keep at rest in a position comfortable for breathing. If in eyes: rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing. Storage statementsStore this substance in a well-ventilated place and keeping container tightly closed. [1] |
|
PHYSICO-CHEMICAL PROPERTIES |
|
|
Molecular weight |
17.031 g/mol [2] |
|
Bulk density/Specific gravity |
0.696 g/L (liquid), Relative vapour density (air = 1): 0.60 [2] |
|
pH |
|
|
Particle size |
|
|
EC |
|
|
Melting/Freezing point |
-77.15 °C at 101 325 Pa [1] |
|
Boiling point |
-33.15 °C at 101 325 Pa [1] |
|
Flash point |
132 °C - closed cup [2] |
|
Flammability |
Flammable (100%) |
|
Vapour density |
|
|
Vapour pressure |
614.9 - 1 003 kPa @ 10 - 25 °C [1] |
|
Solubility in water |
482 g/L @ 25 °C [1] |
|
Solubility in organic solvents |
|
|
Solubility in inorganic solvents |
|
|
Hydrolysis |
|
|
Ionicity in water |
|
|
Surface tension |
23.4 dynes/cm at 11.1 °C; 18.1 dynes/cm at 34.1 °C [2] |
|
Dispersion properties |
|
|
Explosiveness |
Non-explosive (100%) [1] |
|
Other properties |
Autoflammability / self-ignition at 101 325 Pa: 650.85 °C [1] Non-oxidising (100%) [1] Oxidation reduction potential at 20 °C: 3 090 mV [1] Viscosity: 0.255 - 0.475 [1] |
|
Stability and reactivity |
|
|
Chemical stability |
Stable under recommended storage conditions. [2] |
|
Reactivity hazards |
When heated to decomposition, it emits toxic fumes and nitrogen oxides. [2] |
|
Corrosivity |
Corrosive to copper and galvanized surfaces [2] |
|
Polimerization |
|
|
Incompatibility with various substances |
|
|
Special remarks on reactivity |
Dissociation constant: 9.25 @ 25 °C [1] |
|
Physical, chemical and biological coefficient |
|
|
Koc |
|
|
Kow |
Log Kow (Log Pow): 0.23 @ 20 °C [1] |
|
pKa |
pKa = 9.25 at 25 °C [2] |
|
log Kp |
|
|
Henry-constant |
0.507 - 1.621 Pa m³/mol @ 5 - 25 °C [1] |
|
ENVIRONMENTAL FATE AND BEHAVIOUR |
|
|
Artificial pollution sources |
Release to the environment of this substance can occur from industrial use: as processing aid, in processing aids at industrial sites, in the production of articles, of substances in closed systems with minimal release and as an intermediate step in further manufacturing of another substance (use of intermediates). [1] |
|
General terrestrial fate |
It is expected to move slowly through soil. It will be broken down by microorganisms. [2] |
|
General aquatic fate |
|
|
General atmospheric fate |
If ammonia is released to the environment, it will react with other chemicals in air to form small particles that eventually fall to the ground. It can dissolve in water present in the air to form fog. It will move into air from moist soil and water surfaces. [2] |
|
General persistence and degradability |
|
|
Abiotic degradation and metabolites |
|
|
Biodegradation and metabolites |
Readily biodegradable in water (100%) [1] |
|
Bioconcentration |
|
|
Volatilization |
|
|
Photolysis |
|
|
Hydrolysis |
|
|
Soil adsorption and mobility |
|
|
ENVIRONMENTAL CONCENTRATIONS |
|
|
Measured data |
|
|
ECOTOXICOLOGICAL INFORMATION |
|
|
General adverse effects on ecosystem |
|
|
Acute toxicity (LC50, EC50) |
|
|
Aquatic systems |
LC50: 68 µg/L (freshwater fish, 4 days) [1] EC50 / LC50: 110 mg/L (freshwater invertebrates, 48 h) [1] EC50: 2700 mg/L (freshwater algae, 18 days) [1] |
|
Terrestrial systems |
|
|
Chronic toxicity (NOEC, LOEC) |
|
|
Aquatic systems |
EC10 / LC10 or NOEC: 790 µg/L (freshwater invertebrates) [1] |
|
Terrestrial systems |
|
|
HUMAN HEALTH EFFECTS and PROTECTION |
|
|
Routes of human exposures |
Studies suggest that ammonia can be absorbed by the inhalation and oral routes of exposure, but there is less certainty regarding absorption through the skin. Absorption through the eye has been documented. Most of the inhaled ammonia is retained in the upper respiratory tract and is subsequently eliminated in expired air. Almost all of the ammonia produced endogenously in the intestinal tract is absorbed. Exogenous ammonia is also readily absorbed in the intestinal tract. [2] |
|
General effects |
|
|
Endocrine disruption |
|
|
Mutagenicity |
|
|
Carcinogenicity |
|
|
Reprotoxicity |
|
|
Teratogenicity |
|
|
Skin, eye and respiratory irritations |
Skin: Adverse effect observed (corrosive) Eye: Adverse effect observed (irritating) Respiratory: Adverse effect observed (irritating) [1] Skin sensitisation: No adverse effect observed (not sensitising) Respiratory sensitisation: No adverse effect observed (not sensitising) [1] |
|
Metabolism: absorption, distribution & excretion |
Absorption values: Dermal: 10 % [1] |
|
Exposure limits |
DNEL: 47.6 mg/m³ (workers, inhalation, long term, systemic effects, repeated dose toxicity) [1] DNEL: 47.6 mg/m³ (workers, inhalation, acute/short term, systemic effects, repeated dose toxicity) [1] DNEL: 14 mg/m³ (workers, inhalation, long term, local effects, irritation (respiratory tract)) [1] DNEL: 36 mg/m³ (workers, inhalation, acute/short term, local effects, irritation (respiratory tract)) [1] DNEL: 6.8 mg/kg bw/day (workers, dermal, long term, systemic effects, repeated dose toxicity) [1] DNEL: 6.8 mg/kg bw/day (workers, dermal, acute/short term, systemic effects, repeated dose toxicity) [1] DNEL: 23.8 mg/m³ (general population, inhalation, long term, systemic effects, repeated dose toxicity) [1] DNEL: 23.8 mg/m³ (general population, inhalation, acute/short term, systemic effects, repeated dose toxicity) [1] DNEL: 2.8 mg/m³ (general population, inhalation, long term, local effects, irritation (respiratory tract)) [1] DNEL: 7.2 mg/m³ (general population, inhalation, acute/short term, local effects, irritation (respiratory tract)) [1] DNEL: 68mg/kg bw/day (general population, dermal, long term, systemic effects, repeated dose toxicity) [1] DNEL: 68 mg/kg bw/day (general population, dermal, acute/short term, systemic effects, repeated dose toxicity) [1] DNEL: 6.8mg/kg bw/day (general population, oral, long term, systemic effects, repeated dose toxicity) [1] DNEL: 6.8 mg/kg bw/day (general population, oral, acute/short term, systemic effects, repeated dose toxicity) [1] |
|
Drinking water MAC |
|
|
Other information |
|
|
Animal toxicity data |
|
|
Acute toxicity (LD50) |
LD50: 350 mg/kg bw (rat, oral) (toxic) [1] LC50 (60 min) 9.85 - 13.77 mg/L air (rat) (harmful) [1] LC50 (40 min) 14.17 mg/L air (rat), (harmful) [1] LC50 (20 min) 19.96 mg/L air (rat), (harmful) [1] LC50 (10 min) 28.13 mg/L air (rat), (harmful) [1] |
|
Chronic toxicity (NOEL, LOEL) |
NOAEL (rat): 250 - 1 500 mg/kg bw/day (oral) [1] LOAEL (rat): 750 - 1 500 mg/kg bw/day (oral) [1] LOAEL (rat): 105 - 175 mg/m³ air (inhalation)[1] LOEL (guinea pig): 119 mg/m³ air (inhalation) [1] NOAEC (rat): 35 mg/m³ air (inhalation) [1] |
|
ENVIRONMENTAL STANDARDS AND REGULATIONS |
|
| REACH/CLP |
Danger! According to the harmonised classification and labelling (CLP00) approved by the European Union, this substance causes severe skin burns and eye damage, is toxic if inhaled, is very toxic to aquatic life and is a flammable gas. Additionally, the classification provided by companies to ECHA in REACH registrations identifies that this substance is toxic to aquatic life with long lasting effects. Additionally, the classification provided by companies to ECHA in CLP notifications identifies that this substance causes serious eye damage. [1]
According to REACH registrations: H400: Very toxic to aquatic life. H221: Flammable gas. H331: Toxic if inhaled. H314: Causes severe skin burns and eye damage. H411: Toxic to aquatic life with long-lasting effects. [1]
According to CLP notifications: H400: Very toxic to aquatic life. H221: Flammable gas. H331: Toxic if inhaled. H314: Causes severe skin burns and eye damage. H411: Toxic to aquatic life with long-lasting effects. H318: Causes serious eye damage. H280: Contains gas under pressure; may explode if heated. H410: Very toxic to aquatic life with long-lasting effects. H336: May cause drowsiness or dizziness. [1] |
|
EINECS regulation |
̵listed on EINECS (European INventory of Existing Commercial chemical Substances) List [1] |
|
OSHA regulations etc. |
|
|
OTHER INFORMATION, SPECIAL REMARKS |
|
|
Classification and proposed labelling with regard to toxicological data |
|
|
CREATED, LAST UPDATE |
|
|
Created |
2019. 12. 10 |
|
Last update |
2020. 05. 11 |
|
REFERENCES |
|
|
[1] ECHA, Ammonia, anhydrous, https://echa.europa.eu/hu/brief-profile/-/briefprofile/100.028.760, Accessed 2019. 12. 10 [2] Pubchem, Ammonia, https://pubchem.ncbi.nlm.nih.gov/compound/Ammonia, Accessed 2019. 12. 10 |
|