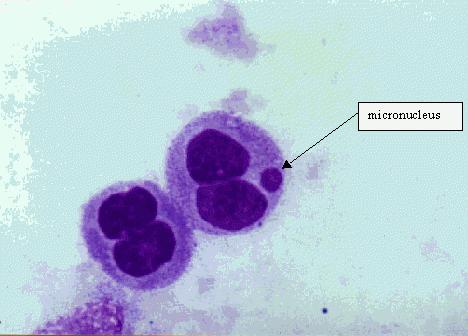
Cell cultures are exposed to the test substances both with and without an exogenous source of metabolic activation unless primary cells with metabolizing capability are used. After exposure to the testsubstance, cell cultures are grown for a period sufficient to allow chromosome or spindle damage to lead to the formation of micronuclei in interphase cells and to trigger the aneuploidy sensitive cell stage (G2/M). Harvested and stained interphase cells are then analysed microscopically for the presence of micronuclei. Ideally, micronuclei should only be scored in those cells that have completed nuclear division following exposure to the test chemical. In cultures that have been treated with a cytokinesis blocker, this is achieved by scoring only binucleate cells. In the absence of a blocker, it is important to demonstrate that the majority of mononucleate cells are likely to have undergone at least one cell division since exposure to the test substance. For all protocols, it is important that cell proliferation is demonstrated in both control and treated cells, together with an assessment of cytotoxicity in the treated cells scored for micronuklei.
Exogenous metabolising systems are required when using cell cultures with inadequate endogenous metabolic capacity. The most commonly used system is a co-factor-supplemented postmitochondrialfraction (S9) prepared from the livers of rodents treated with enzyme-inducing agents such as Aroclor 1254 or preferably a combination of phenobarbitone and β-naphthoflavone.
Cytotoxicity should be determined with and without metabolic activation concurrently in the main experiment. Since micronucleus expression is dependent on cell proliferation, quantification of cellproliferation and cell death should be carried out to obtain a sound evaluation of cell kinetics and micronucleus frequencies. Assessing cytotoxicity as measured by mitotic index is therefore a sub-optimalchoice as mitotic figures may result from mitotic block.