CHEMICAL SUBSTANCE DATASHEET
|
CHEMICAL SUBSTANCE IDENTIFICATION |
|
|
Chemical name |
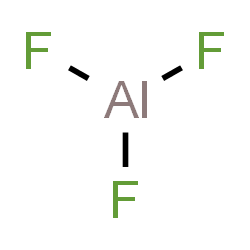
Aluminium fluoride[1] |
|
Synonyms |
Aluminium trifluoride [1] |
|
IUPAC name |
aluminium(3+) trifluoride [1] |
|
CAS No |
7784-18-1 |
|
REACH registration number |
fully registered |
|
EC No |
232-051-1 |
|
Molecular formula |
AlF3 |
|
Substance group/chemical family |
mono-constituent substance/inorganic |
|
Appearance Physical state Odour Form Colour |
solid @ 20°C and 1013 hPa odourless powder white |
|
USES AND HANDLING ISSUES |
|
|
Relevant identified uses |
manufacture of basic metals, including alloys uses in aluminium industry and casting industry as flux uses in aluminium alloys uses as agent of mineralizing in industrial process uses in ceramic industry manufacture of abrasives production of glassware inhibitors of fermentation catalyst in chemical reactions and organic synthesis [1] |
|
Handling considerations |
Handling: Handle the product so that formation of dust is at a minimum. Ensure adequate ventilation. Do not eat or smoke while using the product. Avoid contact with eyes and skin. Personal hygiene is important. |
|
PHYSICO-CHEMICAL PROPERTIES |
|
|
Molecular weight |
83.976 g/mol [1] |
|
Bulk density/Specific gravity |
3.1 g/cm3 Information regarding density of aluminum fluoride was sourced from various handbooks or review articles including CRC Handbook of Chemistry and Physics (81st Ed.), Dangerous Properties of Industrial Materials (7thEd.), and the Agency for Toxic Substances and Disease Registry (ATSDR). However, Dangerous Properties of Industrial Materials and ATSDR both cite secondary sources that are not peer-reviewed, and as such, are not considered valid. Based on the primary information sourced from CRC Handbook of Chemistry and Physics, the density of aluminum fluoride was reported as 3.1 g/cm3 (corresponding to be 3100 g/L) at room temperature. [1] |
|
pH |
|
|
Particle size |
Average size diameter (key study): 20, 90 and 112 µm depending on the source of the test article. Mass Median Diameter (supporting study): Inhalable fraction (less than 100 µm): 47.9%; Thoracic fraction (less than 10 µm): > 26.8%; Respirable fraction (less than 4 µm): < 1%. [1] |
|
EC |
|
|
Melting point |
1291°C Information regarding melting/freezing point of aluminum fluoride was sourced from CRC Handbook of Chemistry and Physics (81st Ed.), known to contain peer-reviewed data of Klimisch-2 reliability, which reports that the test substance sublimes at 1276°C. However, based on the information obtained from secondary sources such as Dangerous Properties of Industrial Materials (7thEd.) and the Agency for Toxic Substances and Disease Registry (ATSDR) the test substance melts at 1291°C. These data, although conflicting, both agree that given the high temperatures at which the test substance sublimes or melts, it is considered to be a non-volatile solid. Additionally, considering that the data obtained from the secondary sources are non-peer-reviewed and of Klimisch-4 reliability, they have not been included in this assessment. [1] |
|
Boiling point |
1537°C Information regarding boiling point of aluminum fluoride was sourced from CRC Handbook of Chemistry and Physics (81st Ed.), known to contain peer-reviewed data of Klimisch-2 reliability, which reports that the test substance sublimes at 1276°C prior to boiling. This is consistent with the information reported in the secondary source, Agency for Toxic Substances and Disease Registry (ATSDR). Conversely, based on the information obtained from the secondary source, Dangerous Properties of Industrial Materials (7th Ed.), the test substance boils at around 1537°C, while no information on the atmospheric pressure was reported. These data, although conflicting, all agree that given the high temperatures at which the test substance sublimes or boils, it is considered to be a non-volatile solid. Additionally, considering that the data obtained from the secondary sources are non-peer-reviewed and of Klimisch-4 reliability, they have not been included in this assessment. [1] |
|
Flash point |
In accordance with column 2 of REACH (Regulation (EC) No 1907/2006) Annex VII, the flash point study (required in section 7.9) does not need to be conducted as the substance is inorganic. [1] |
|
Flammability |
non flammable The notifiable substance is not flammable when in contact with water nor does it possess pyrophoric properties. As well, it has almost no vapour pressure, a non-existent flash point, and based on thermogravimetric analysis, is not ignitable. Based on this information, the data are conclusive but not sufficient for classification. [1] |
|
Vapour density |
|
|
Vapour pressure |
In accordance with column 2 of REACH (Regulation (EC) No 1907/2006) Annex VII, the vapour pressure study (required in section 7.5) does not need to be conducted as the melting point is above 300ºC. [1] |
|
Solubility in water |
5.3 mg/L to 9.4 mg/L
The solubility of aluminum fluoride (AlF3) was measured using flask method and was reported based on Al and F, ranging from 5.3 mg/L to 8.5 mg/L of AlF3 on the basis of F analysis and 5.4 mg/L to 9.4 mg/L of AlF3 on the basis of Al analysis.The solubility of aluminum fluoride was also sourced from secondary and non-peer reviewed sources of Klimisch-4 reliability. However, since these endpoints cannot be associated with the form of Aluminium Fluoride produced by the standard (wet & dry) manufacturing methods as reflected in Endpoint study record: Water solubility.001 - key ¿ 2002, they are not considered as valid. [1] |
|
Solubility in organic solvents |
|
|
Solubility in inorganic solvents |
|
|
Hydrolysis |
In the aquatic environment, the substance is expected to hydrolyse to hydroxy-aluminium species. [1] |
|
Ionicity in water |
|
|
Surface tension |
In accordance with column 2 of REACH (Regulation (EC) No 1907/2006) Annex VII, the surface tension study (required in section 7.6) does not need to be conducted as based on structure, the surface activity is neither expected nor predicted, and surface activity is not a desired property of the material. [1] |
|
Dispersion properties |
|
|
Explosiveness |
non explosive As there are no chemical groups associated with explosive properties present in the notifiable substance, the data are conclusive but not sufficient for classification. [1] |
|
Other properties |
In accordance with column 2 of REACH (REGULATION (EC) No 1907/2006) Annex VII, the auto flammability study (required in section 7.12) does not need to be conducted as the preliminary results exclude self-heating of the substance up to 400ºC. [1] |
|
Stability and reactivity |
|
|
Chemical stability |
Stable under normal conditions. Avoid high temperatures (over 600°C in dry state and 300°C in moist condition), except in professional use. [1] |
|
Reactivity hazards |
Aluminium fluoride slowly dissolves in strong sulfuric acid with the release of hydrogen fluoride, and in strong alkaline water solutions, aluminat is formed. Aluminium fluoride is slowly decomposed by melted alkalies, with the formation of fluorides and aluminat. [1] |
|
Corrosivity |
|
|
Polimerization |
|
|
Incompatibility with various substances |
Avoid contact with strong oxidizing agents, acids and water. Aluminium fluoride slowly dissolves in strong sulfuric acid with the release of hydrogen fluoride, and in strong alkaline water solutions, aluminat is formed. Aluminium fluoride is slowly decomposed by melted alkalies, with the formation of fluorides and aluminat. [1] |
|
Special remarks on reactivity |
no oxidising properties The substance is incapable of reacting exothermically with combustible materials, for example on the basis of the chemical structure (e.g. organic substances not containing oxygen or halogen atoms and these elements are not chemically bonded to nitrogen or oxygen. [1] When the product is heated, it decomposes and toxic gas of hydrogen fluoride can form, especially when water is present. [1] |
|
Physical, chemical and biological coefficient |
|
|
Koc |
|
|
Kow |
In accordance with column 2 of REACH (Regulation (EC) No 1907/2006) Annex VII, the partition coefficient study (required in section 7.8) does not need to be conducted as the substance is inorganic. [1] |
|
pKa |
5.5 to 7. [1] Information regarding dissociation constants was sourced from a literature article reporting computer-aided QSAR estimates of the speciation of the substance in aqueous media utilizing SOLGASWATER (version Win SGW) model. The author concluded that the substance would dissociate to release fluoride ions and/or exchange fluoride for hydroxide, depending on both the pH and the fluoride concentration. Low pH (i.e. < pH ~6) and higher fluoride concentrations would favour more highly fluorinated/less highly hydroxylated species, and higher pH (i.e. > pH ~6) and lower fluoride concentrations would favour less highly fluorinated/more highly hydroxylated species. The author goes on to state that the pKa values defined according to the reactions Al(OH)mFn(3 -m-n)++ H2O = Al(OH)m+1Fn(2 -m-n)++ H+ [1] |
|
log Kp |
|
|
Henry-constant |
Information on Henry¿s Law constant and distribution modelling are not required under REACH, and no other distribution data is available. [1] |
|
ENVIRONMENTAL FATE AND BEHAVIOUR |
|
|
Artificial pollution sources |
|
|
General terrestrial fate |
|
|
General aquatic fate |
In the aquatic environment, the substance is expected to hydrolyse to hydroxy-aluminium species. No rate information is available on this hydrolysis pathway, but it would be expected to be sufficiently rapid to prevent the substance from persisting in environmental waters. [1] |
|
General atmospheric fate |
|
|
General persistence and degradability |
The substance does not fulfil any of the screening criteria for persistence, bioaccumulation or toxicity and hence is not a PBT or vPvB. [1] |
|
Abiotic degradation and metabolites |
In the aquatic environment, the substance is expected to hydrolyse to hydroxy-aluminium species. [1] |
|
Biodegradation and metabolites |
not applicable, the substance is inorganic |
|
Bioconcentration |
Due to the dissociation behaviour of AlF3, its bioaccumulation cannot be assessed. [1] The substance does not fulfil any of the screening criteria for persistence, bioaccumulation or toxicity and hence is not a PBT or vPvB. [1] |
|
Volatilization |
|
|
Photolysis |
|
|
Hydrolysis |
In the aquatic environment, the substance is expected to hydrolyse to hydroxy-aluminium species. No rate information is available on this hydrolysis pathway, but it would be expected to be sufficiently rapid to prevent the substance from persisting in environmental waters. [1] |
|
Soil adsorption and mobility |
|
|
ENVIRONMENTAL CONCENTRATIONS |
|
|
Measured data |
|
|
ECOTOXICOLOGICAL INFORMATION |
|
|
General adverse effects on ecosystem |
|
|
Acute toxicity (LC50, EC50) |
|
|
Aquatic systems |
In a series of studies to test for the potential aquatic toxicity of aluminium fluoride (AlF3) it was only slightly soluble in aqueous solution at a loading concentration of 1000 mg/L. Therefore, in each study, the undiluted water phase from the AlF3 mixture was set to 100% v/v, which was used as the high-concentration for testing in studies conducted in fish, daphnids, and algae. Dilutions also were tested in algae and microorganisms. [1] AlF3 was reported to have low acute toxicity to fish, an aquatic invertebrate species, to algae and to microorganisms derived from activated sludge. [1] |
|
Terrestrial systems |
In accordance with column 2 of REACh (Regulation (EC) No 1907/2006) Annexes IX and X, the effects on terrestrial toxicity do not need to be investigated based on the findings of the Chemical Safety Assessment; the substance does not fulfill classification criteria according to the applicable regulations and does not fulfill the criteria for vPvB or PBT. [1] |
|
Chronic toxicity (NOEC, LOEC) |
|
|
Aquatic systems |
In accordance with column 2 of REACh (Regulation (EC) No 1907/2006) Annex IX, the long-term toxicity testing on fish, invertebrates (required in section 9.1.5) does not need to be conducted based on the findings of the Chemical Safety Assessment; the substance does not fulfill classification criteria according to the applicable regulations and does not fulfill the criteria for vPvB or PBT. [1] |
|
Terrestrial systems |
In accordance with column 2 of REACh (Regulation (EC) No 1907/2006) Annexes IX and X, the effects on terrestrial toxicity do not need to be investigated based on the findings of the Chemical Safety Assessment; the substance does not fulfill classification criteria according to the applicable regulations and does not fulfill the criteria for vPvB or PBT. [1] |
|
HUMAN HEALTH EFFECTS and PROTECTION |
|
|
Routes of human exposures |
inhalation, dermal, eyes, ingestion |
|
General effects |
Acute: respiratory irritation, possible nose bleeding or vomiting: Chronic: aggravates bronchitis/ asthma; increased bone density. [2] |
|
Endocrine disruption |
|
|
Mutagenicity |
|
|
Carcinogenicity |
Not classifiable as a human carcinogen. (Fluorides as F) [2] Not classifiable as a human carcinogen. (Aluminum metal and insouble compound) [2] A carcinogenicity study is not required as there is no evidence from the 28-day and 5-month inhalational repeat dose toxicity study findings that exposure to the notifiable substance results in hyperplasia or pre-neoplastic lesions, and the notifiable substance is not classified as a genotoxic compound. As such, the notifiable substance is not classified as carcinogenic as data are lacking. [1] |
|
Reprotoxicity |
The substance is not classified for reproductive toxicity as data are conclusive but not sufficient for classification. [1] |
|
Teratogenicity |
|
|
Skin, eye and respiratory irritations |
The available in vivo studies for eye and skin irritation indicate that the notifiable substance is not irritating to the eyes or skin. Thus, the data are conclusive but not sufficient for classification for eye and skin irritation. [1] No information is available for respiratory irritation, thus the notifiable is not classified as a respiratory irritant as data are lacking. [1] Anhydrous aluminum fluoride is a strong irritant to tissue. [2] Chronic fluorosis generally develops after prolonged (10-20 years) exposure to industrial dusts, insecticides, or water where fluorides exceed 3 to 4 ppm. This is especially true in workers involved in the production of aluminum, steel, or glass. [2] |
|
Metabolism: absorption, distribution & excretion |
|
|
Exposure limits |
Workers (systemic effects) (inhalation route): DNEL (Derived No Effect Level): 0.047 mg/m³ Workers (systemic effects) (dermal route): DNEL (Derived No Effect Level): 0.068 mg/kg bw/day
General population (systemic effects) (inhalation route): DNEL (Derived No Effect Level): 0.008 mg/m³ General population (systemic effects) (dermal route): DNEL (Derived No Effect Level): 0.024 mg/kg bw/day General population (systemic effects) (oral route): DNEL (Derived No Effect Level): 0.002 mg/kg bw/day [1]
|
|
Drinking water MAC |
|
|
Other information |
There is no information available concerning respiratory sensitisation. [1] |
|
Animal toxicity data |
|
|
Acute toxicity (LD50) |
LD50 : 103 mg/kg (mouse, oral) [2] |
|
Chronic toxicity (NOEL, LOEL) |
Information on the effects of aluminum fluoride on reproduction can be obtained from a two-generation reproductive toxicity study in rats conducted with the read-across substance trisodium hexafluoroaluminate (cryolite). The reproductive effects of cryolite was assessed in a two-generation dietary study in rats, equivalent in design to OECD test guideline 416 (GLP-compliance not reported). A NOAEL of 42 mg/kg bw/day (600 ppm) was identified for reproductive toxicity due to decreased pup body weights at higher dose levels. A NOAEL for parental toxicity could not be established due to findings of dental fluorosis in treated animals. [1]
NOAEL (teratogenicity): 100 mg/kg bw/day (oral, mice) Information on the effects of aluminum fluoride on developmental toxicity can be obtained from a prenatal toxicity study conducted with the read-across substance trisodium hexafluoroaluminate (cryolite). The effects of cryolite on development were evaluated in an oral gavage prenatal development study in mice, equivalent to OECD Test Guideline 414 (GLP status unknown) (Nemec, 1991) Since effects on the fetuses were noted at severely maternal toxic doses, there is no support that the read-across substance (cryolite) was teratoginic in mice. [1] |
|
ENVIRONMENTAL STANDARDS AND REGULATIONS |
|
|
REACH/CLP |
No signal word The substance does not fulfil any of the screening criteria for persistence, bioaccumulation or toxicity and hence is not a PBT or vPvB. [1] |
|
EINECS regulation |
̵ |
|
OSHA regulations etc. |
|
|
|
|
|
OTHER INFORMATION, SPECIAL REMARKS |
|
|
Classification and proposed labelling with regard to toxicological data |
|
|
Disposal considerations |
Removal of remains and waste: Not classified as hazardous waste. Consult waste disposal method with responsible authority and local regulations. Waste should be disposed in a responsible manner and delivered to an authorized waste station. |
|
CREATED, LAST UPDATE |
|
|
Created |
2020.05.27 |
|
Last update |
2020.06.05 |
|
REFERENCES |
|
|
[1] ECHA, Aluminium fluoride, https://echa.europa.eu/hu/registration-dossier/-/registered-dossier/15258, Accessed 2020.05.26 [2] PUBCHEM, Aluminium fluoride, https://pubchem.ncbi.nlm.nih.gov/compound/Aluminum-fluoride#section=Human-Toxicity-Excerpts, Accessed 2020.06.05 |
|